If you need to print this PDF or save for later
click below to download your copy.
[button size=”medium” style=”secondary” text=”Download PDF” link=”https://vetovationnew.instawp.xyz/wp-content/uploads/2019/06/2019-Simplified-Minimally-Invasive-Surgical-Approach-for-Prophylactic-Laparoscopic-Gastropexy-in-21-Cases.pdf” target=”none”]
Simplified Minimally Invasive Surgical Approach for Prophylactic Laparoscopic Gastropexy in 21 Cases
Claire Deroy, DVM, MSCs, Harriet Hahn, DVM, Camille Bismuth, DVM, MSCs, DECVS, Guillaume Ragetly, PhD, DVM, DACVS-DECVS, Eymeric Gomes, DVM, ECVDI, Cyrill Poncet, DVM, DECVS
Abstract
The objective of this study was to describe the operative technique and outcome of a simplified laparoscopic gastropexy approach in dogs. Twenty-one dogs undergoing prophylactic laparoscopic gastropexy with a simple continuous barbed suture without incising the seromuscular layer of the stomach and transversus abdominis muscle were reviewed. In 20 cases, additional procedures were performed (18 ovariectomies and 2 prescrotal castrations); 1 dog had two prior episodes of gastric dilation without volvulus and underwent gastropexy with a prophylactic intent. The gastropexy procedure had a median duration of 33 min (range 19–43 min). V-Loc 180 absorbable and the V-Loc PBT nonabsorbable suturing devices were used in 8 and 13 dogs, respectively. Minor intraoperative complications occurred in four cases: broken suture (1), needle dislodgement (2), and folded needle (1). Minor complications included self-limiting wound complications (3), abdominal discomfort (2), vomiting (1), and inappetence (2). Postoperative abdominal ultrasound performed after a median of 8 mo (6–36 mo) confirmed permanent adhesion at the gastropexy site in all dogs. One dog developed a fistula (1 yr postoperatively) and another a granuloma (3 mo postoperatively), both at the gastropexy site. Prophylactic laparoscopic gastropexy may be performed with knotless unidirectional barbed suture without creating an incision on the abdominal wall and stomach. (J Am Anim Hosp Assoc 2019; 55:152–159. DOI 10.5326/JAAHA-MS-6879)
Introduction
Introduction Gastric dilatation and volvulus (GDV) is a life-threatening syndrome reported most commonly in large- and giant-breed, deep-chested dogs. Despite rapid diagnosis and early surgical intervention, GDV can lead to devastating outcomes. However, GDV can be prevented in predisposed dogs by a prophylactic gastropexy.¹⁻⁵ Prophylactic laparoscopic gastropexy has been traditionally performed with a laparoscopic-assisted technique, relying on multiple- or single-port access, and a small abdominal incision to complete the procedure.¹,³,⁶,⁷ Total laparoscopic gastropexies have been gaining popularity⁴⁻¹⁴ Total laparoscopic gastropexy has been demonstrated to be superior to both open- and laparoscopic assisted methods in terms of decreased postoperative pain.⁴,⁵,¹⁰,¹³ Prophylactic gastropexy can be performed at the time of a routine surgery such as ovariectomy, castration, cryptorchidectomy, exploratory laparotomy for foreign body obstructions, and visceral organ biopsies.³,⁷,¹⁵⁻¹⁷ Laparoscopic suturing, particularly knot-tying, is technically difficult and is considered one of the most challenging and time consuming steps of laparoscopic surgery.¹⁸ The demand for minimally invasive surgical procedures has fostered the development of alternative knotless sutures such as the barbed suture intended to eliminate the need for intracorporeal knot-tying and the development of intracorporeal suturing devices. The barbed suture creates multiple anchor points to distribute tension along the suture line and achieves strength through knotless anchoring within tissue.⁴,¹¹,¹⁹
From Clinique Veterinaire Alliance, Bordeaux, France (C.D.); and Center Hospitalier Vétérinaire Fregis, Acrueil, France (H.H., C.B., G.R., E.G., C.P.).
Correspondence: deroy.claire@hotmail.fr (C.D.)
GDV (gastric dilatation and volvulus)
Accepted for publication: November 9, 2018.
This technique relies on a welded loop at the end of the barbed suture strand, through which the needle may be passed and locked at the beginning of a suture line. Furthermore, the biomechanical strength of barbed suture during laparoscopic gastropexy has been found to be similar to or higher than intracorporeal knot-tying with standard suture.¹¹,¹²
The canine total laparoscopic gastropexy has previously been described using one or two simple continuous barbed suture lines between the incised seromuscular layer of the stomach and the transversus abdominis muscle.⁴,⁵,⁹,¹³,¹⁴ Most gastropexies rely on healing of the sutured incisions, which creates permanent attachment. The incision should be carefully performed so that the submucosal layer of the stomach is not penetrated. Instead of making an incision, the use of monopolar electrosurgery to abrade the peritoneum at the proposed gastropexy sites on the body wall and the stomach has also been reported.⁹,¹⁴ The incisions or the abrasion of the peritoneum and the gastric wall add some surgical trauma and increase surgery time; however, little is known about the need for those.
The objectives of this study were to (1) describe the efficacy of intracorporeal suturing with knotless unidirectional barbed sutures using one simple continuous suture line without making an incision or an abrasion through the seromuscular layer of the stomach and the transversus abdominis muscle in client-owned dogs undergoing total laparoscopic gastropexy and (2) report the short- and long-term outcomes and intraoperative and postoperative complications. We hypothesized that this procedure is safe and creates a lasting gastropexy.
Materials and Methods
Medical records of client-owned dogs undergoing prophylactic laparoscopic gastropexy using a single simple continuous barbed suture line without incising the seromuscular layer of the stomach and the transversus abdominis muscle between February 2014 and May 2017 were reviewed. Only complete records with signalment, history, physical examination findings, procedural information, surgery time, performance of other laparoscopic or extraabdominal procedures, length of hospitalization, intra- and postoperative complications, and a minimum 6 mo clinical follow-up were included. The inclusion criterion consisted of the use of one simple continuous knotless unidirectional barbed suture line without creating an incision on the abdominal wall or the stomach.
Dogs were excluded if there were insufficient data for all required medical parameters, if the available postoperative follow-up was shorter than 6 mo, and if a postoperative abdominal ultrasound examination had not been performed.
Each dog underwent a complete physical examination and a preanesthetic bloodwork before surgery to confirm general health status and detect any abnormalities that would preclude general anesthesia.
Surgical Techniques
Each dog was fasted 12 hr prior to surgery. Dogs were premedicated and standard anesthetic and analgesic protocols were selected on a case-by-case basis at the time of surgery at the discretion of the attending anesthesiologist. General anesthesia was maintained with isoflurane in 100% oxygen, via endotracheal tube, to effect. Each dog received prophylactic antibiotic (cefazolin 20 mg/kg IV) 30 min prior to the first skin incision. Dogs were monitored via electrocardiography, blood pressure and body temperature measurements, and capnography.
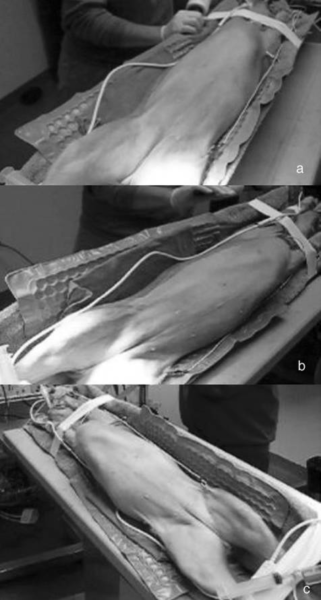
Dogs were initially placed in dorsal recumbency and prepared for aseptic surgery. A reverse Trendelenburg position was used.
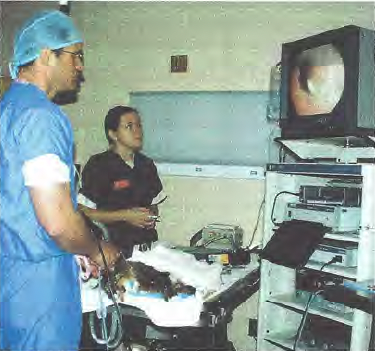
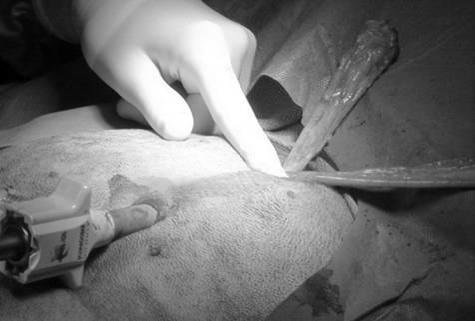
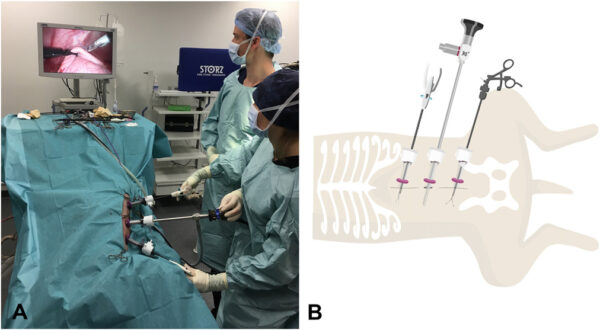
Laparoscopic gastropexy surgeries were carried out by board certified specialists in surgery or by a third-year surgery resident directly supervised by a board-certified surgeon. The main surgeon stood on the patient’s left side, with the endoscopy veterinary tower located cephalad of the dog’s head (Figure 1A).
All laparoscopic gastropexies were performed through three ports placed on the ventral midline inserted via a modified Hasson approach. Following the insertion of the first portᵃ ~1 cm caudal to the umbilicus, the peritoneal cavity was insufflated with CO2 by means of a pressure-regulating mechanical insufflatorᵇ to a maximum pressure of 10 mm Hg. Under direct observation, two additional instrument 10 mm ports were placed approximately 10 cm cranially and caudally to the camera port along the linea alba.
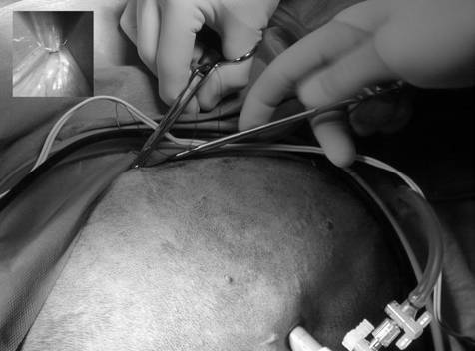
Once all ports were established, the abdomen was explored, and a 10 mm laparoscopic Babcock grasping forcepsᶜ or a 10 mm laparoscopic Dorsey grasping forcepsᶜ was introduced through the caudal instrument port to allow manipulation of the stomach (Figure 1B).
An avascular region of the pyloric antrum was grasped with Babcock or Dorsey forceps midway between the greater and the lesser curvatures. Incisions were performed in neither the transversus abdominis muscle nor the pyloric antrum. The dog was rolled to the left (Figure 1A) and the intra-abdominal pressure was reduced to 6– 8 mmHg to facilitate tension-free apposition of the stomach to the body wall. Babcock or Dorsey forceps were used to hold the pyloric antrum in apposition with the abdominal wall.
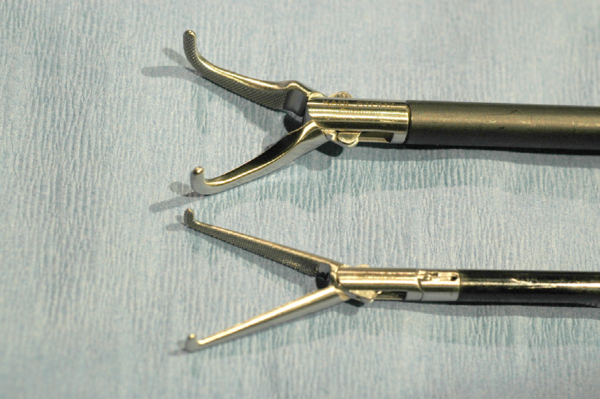
Intracorporeal suturing was performed with a straight automatic endoscopic suturing deviceᵈ introduced through the cranial instrument portal (Figure 1B). The unidirectional 15 cm barbed suture (2-0 V-Loc 180 absorbableᵉ or V-Loc PBT nonabsorbableᵉ) was loaded in a standard fashion into the straight endoscopic suturing device and introduced through the cranial port to perform one simple continuous suture line between the stomach and the right body wall lateral to the rectus abdominis muscle and 2–4 cm caudal to the 13th rib.
The barbed suture was placed in accordance to the manufacturer’s instructions, starting with a bite of the transversus abdominis muscle, prior to penetrating the seromuscular layer of the stomach.²⁰ The tip of the needle was then passed through the eye of the welded loop at the end of the device to anchor the suture line, and the simple continuous pattern was continued. After each completed bite, the suture was pulled taut to bring the gastric wall in contact with the transversus abdominis muscle to secure the suture in the tissue. Extracorporeal pressure was applied through the abdomen by the surgeon’s left hand during suturing to ensure a good bite on the transversus abdominis muscle. The suture was ended with two bites in the transversus abdominis muscle oriented 1808 to one another, and the suture was tensioned so the final barb engaged the tissue preventing backward movement; then, the suture was cut intracorporeally.⁵
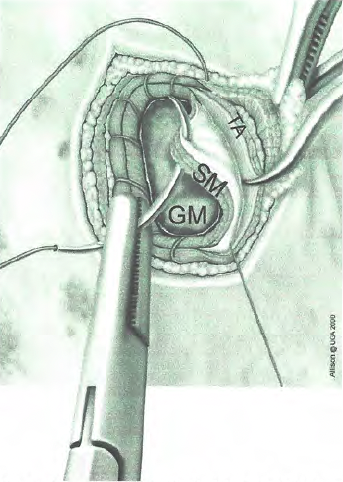
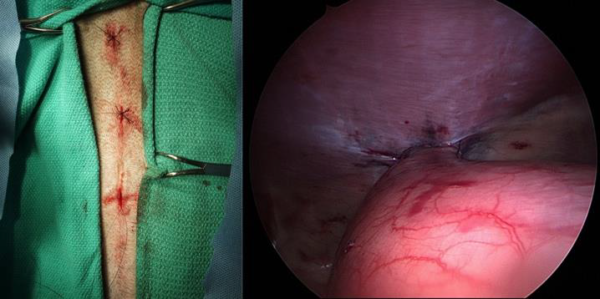
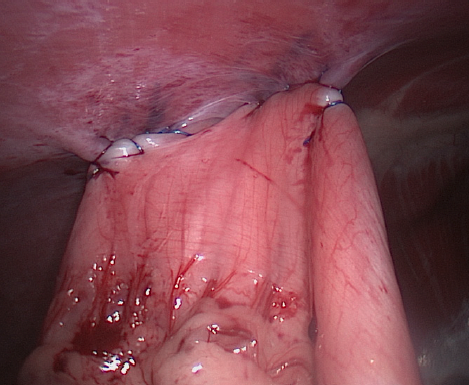
At the end of the procedure, the scope was used to evaluate the gastropexy line (Figure 2), the gastropexy adhesion was defined as adequate if the transverus abdominis muscle could be displaced medially with no suture pullout or tissue tearing during medial traction on the stomach. The ports were removed after abdomen deflation. Port site closure was performed in a standard three-layer manner, that is, a simple continuous suture pattern to appose the external layer of the rectus sheath with 0 or 2-0 poliglecaproneᶠ, followed by closure of the subcutaneous layer with 3-0 or 4-0 poliglecaprone, and then intradermal suture by 3-0 or 4-0 poliglecaprone.
If the owners requested to have an ovariectomy or conventional castration performed, it was done prior to the gastropexy. Each dog was discharged on the day of the surgery with a 3 day supply of meloxicam (0.1 mg/kg orally once a day). Criteria for a safe discharge from the hospital were a good clinical condition and a pain-free dog.
Total surgery time was measured between initial skin incision and end of port site closure. Gastropexy time included time from skin incision to skin closure minus the surgery time of the additional procedure.
Follow-Up
Follow-up information was obtained from medical records and via owner questionnaires conducted by a single investigator. Queries were related to the presence and duration of postoperative clinical signs including lethargy, gastric disturbance, incidence of vomiting/regurgitation, abdominal discomfort, inappetence, wound-related complications, quality of life, weight loss, and presence of any signs of gastric dilation.
Long-term focal postoperative abdominal ultrasound was performed in all cases by a single board-certified radiologist or by a trained European College of Veterinary Diagnostic Imaging resident. The minimum required follow-up time for ultrasound was 6 mo. Dogs were fasted for 12 hr prior to ultrasound imaging. Contact of the pyloric antrum with the peritoneal surface of the abdominal wall was evaluated, as was the presence of suture.¹,⁴,¹⁰,²¹ The pyloric antrum and body wall were assessed for the absence of the slide sign (abdominal viscera moving along the peritoneal lining with respiratory motion and during distal antral contractions) at the site of the gastropexy, indicating adhesion and successful gastropexy.⁵
Descriptive Statistical Analysis
Statistical analysis was performed using a statistical softwareg . Numerical data was reported as “median (range).”
Results
A total of 23 dogs underwent laparoscopic gastropexy using knotless unidirectional barbed sutures during the study period. Two dogs were excluded because long-term postoperative ultrasound scans were not performed. Twenty-one cases met the inclusion criteria. All dogs were purebreeds with breeds including German shepherd dog (6), Great Dane (4), golden retriever (1), Dogue de Bordeaux (1), Belgian Tervuren (1), Newfoundland (1), Cane Corso (1), Beauceron (1), Bernese mountain dog (1), giant schnauzer (1), Picardy spaniel (1), Saint Bernard (1), and Weimaraner (1). The patients comprised 19 intact females and 2 intact males with a median age at the time of the surgery of 12 mo (7–84 mo) and a median weight at the time of the surgery of 35 kg (22–66 kg). Complete physical examinations performed in all dogs were normal except in one dog with a systolic heart murmur. All hematology and serum biochemistry analyses were within the normal range except in one case that revealed von Willebrand disease.
In 20 of the 21 cases, additional procedures were performed including 18 ovariectomies and 2 prescrotal open castrations; 1 dog underwent the gastropexy procedure with a primary intent indicated by two prior episodes of gastric dilation with no volvulus, which had resolved with medical treatment prior to the surgery. The median total duration of surgery including additional procedures was 44 min (35–52 min).
The median duration of laparoscopic gastropexy from skin incision to skin closure was 33 min (19–43 min). The median number of suture bites was 7 (6–9 bites). V-Loc 180 absorbable and the V-Loc PBT nonabsorbable suturing devices were used in 8 and 13 dogs, respectively. Subjective evaluation of gastropexy adherence revealed no observable tearing or suture loosening.
Minor intraoperative complications occurred in four cases, including, suture breakage (1), needle dislodged from the endoscopic suturing device (2), and needle folding (1). Laparoscopic gastropexy was performed using one suture pack in 20 dogs. Only the case with the suture breakage needed two suture packs. No major complication was observed, and no dog required conversion to open laparotomy. All dogs were discharged on the day of the surgery.
Three cases presented minor self-limiting wound-related complications at a single-port site that did not require veterinary intervention and lasted a median of 3 days (2–5 days). Of these three cases, one was diagnosed with incisional inflammation (bruising, erythema) and a small area of poor apposition, one with incisional infection, and one incisional seroma formation that resolved within 3 days.
Two dogs experienced abdominal discomfort during the first 2 postoperative days. Vomiting and loss of appetite were present in one dog, which resolved spontaneously within 2 days.
A minimum of 6 mo follow-up was available for all dogs, with a median of 8 mo (range 6–36 mo). Owners reported excellent health and no complication in 20 cases. There was no vomiting, gastric impairment, or weight loss, and there was no report of gastric dilatation or GDV. One dog experienced inappetence and mild cranial abdominal discomfort 12 mo postoperatively. Abdominal ultrasound revealed a fistula between the subcutaneous layer of the skin and the stomach, along with the presence of the suture (nonabsorbable). Bacteriology assays could not be performed because no tissue or liquid could be aspirated. A 6 wk course of antibiotics was prescribed leading to subsidence of clinical signs and improvement of the lesion at the 6 wk ultrasound recheck.
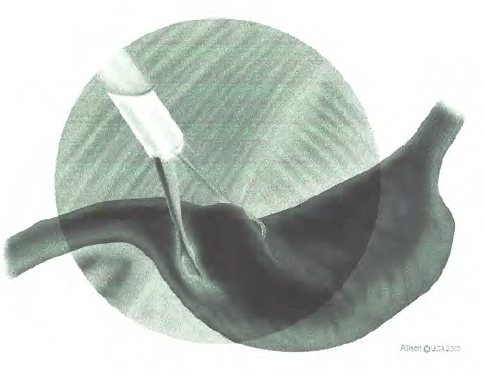
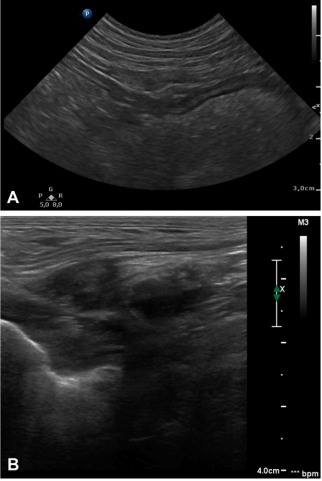
Postoperative abdominal ultrasound performed at a median of 8 mo (6–36 mo) confirmed permanent adhesion formation at the gastropexy site in all dogs (Figure 3A). Eight dogs had a first ultrasound control performed at 3 mo, and permanent adhesion formation was already observed at that early stage in all eight dogs. At 3 mo postoperatively, one dog had a focal thickening of the gastric wall with loss of layering consistent with a granuloma along with the presence of the suture (nonabsorbable) at the gastropexy site without associated clinical signs. This finding was no longer present at the 6 mo ultrasound examination. Ultrasound had a crucial role in diagnosing the fistula that developed at the gastropexy site 12 mo postoperatively, as detailed above (Figure 3B).
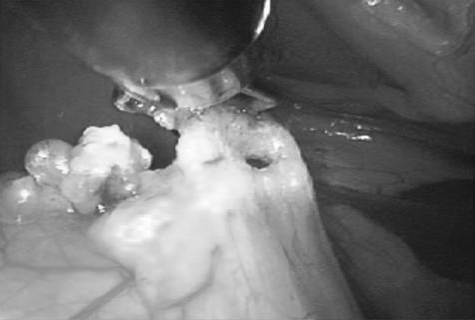
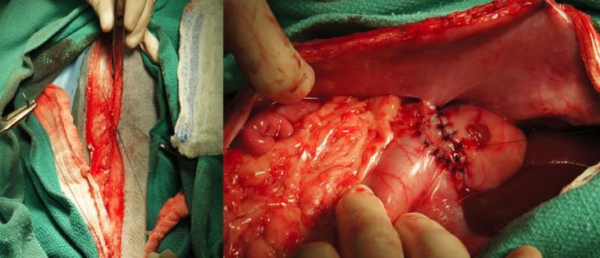
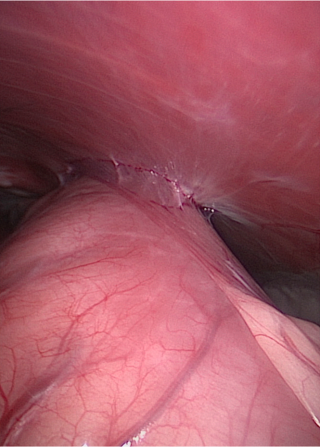
One case underwent veterinary laparoscopy for liver biopsies at 21 mo after the gastropexy procedure, at which point the gastropexy proved intact (Figure 4).
Discussion
The results of the present retrospective case series suggest that the hereby described total laparoscopic gastropexy technique with intracorporeal suturing with knotless unidirectional barbed sutures using one simple continuous suture line without making abrasions or incisions through the seromuscular layer of the stomach and the transversus abdominis muscle is a feasible minimally invasive surgical option for gastropexy in dogs, which results in an intact gastropexy long term.
Total laparoscopic gastropexy using barbed sutures with a welded loop eliminates the need for knot-tying and is consequently less technically challenging, can lead to faster suture placement, and significantly decreases operative times in comparison with older open and total laparoscopic gastropexy techniques.⁴,¹¹,¹² One suture strand of 15 cm was used to complete the gastropexy, and this was long enough to complete a simple continuous suture pattern with at least six bites. However, the minimum number of suture bites required to produce a reliable gastropexy has not yet been determined.⁵ According to Coleman et al., it is easier to work with a short suture strand.⁵ When longer suture material is used, it has a tendency to get caught in the omentum or other surrounding tissue and makes suture manipulation in the abdomen more cumbersome.⁴,⁵
Incisional gastropexy has previously been described as an effective technique that results in a permanent adhesion of the stomach to the body wall without added risks of rib fractures or pneumothorax and only transient gastrointestinal disturbances (e.g., vomiting, regurgitation, diarrhea, inappetence).² All previously described laparoscopic gastropexy techniques have been performed with an incision in the seromuscular layer of the stomach and the transversus abdominis muscle with either veterinary laparoscopy Metzenbaum scissors or harmonic scalpel.⁴,⁵,¹⁰,¹³,¹⁴ In the present study, no incision was performed before gastropexy. Previous studies reported a laparoscopic-assisted gastropexy technique that used monopolar electrosurgery to scarify the peritoneum at the intended gastropexy sites on the body wall and stomach, instead of making an incision through the seromuscular layer of the stomach and the transversus abdominis muscle.⁹,¹⁴ The gastropexy created had comparable biomechanical strength to incisional gastropexy.⁹ Tissue injury to the peritoneum, which requires re-epithelialization, is a prerequisite for adhesion formation.²² The authors think that each of the barbs along the length of the suture engages the tissue, resists against tissue pull out, maintains constant tension, and generates sufficient trauma to promote fibrous adhesion. However, no histopathology or mechanical tests have been performed in this series to confirm that barbs create enough trauma to cause adhesion between the peritoneum and the stomach. Adhesion formation was observed with myometrial closure using barbed suture, and concerns have been raised insofar as potential for increased risk of adhesions or inflammation due to the barbs that are cut into the suture.²³,²⁴ Adhesion formation may also be caused by tissue trauma secondary to tissue manipulation with the instruments during suture placement. Nevertheless, the minimum tissue damage required to form a permanent adhesion in the dog remains unknown.
Acute biomechanical testing of barbed suture has shown similarto-increased biomechanical strength compared with monofilament suture for open incisional gastropexy.¹¹,¹² The maximum tensile strength required for successful gastropexy is unknown. No biomechanical study has been performed to compare incisional, abrasion, and nonincisional gastropexy. Traditionally, incisional open and laparoscopic-assisted gastropexies have been performed with two suture lines that are ~3–4 cm long between seromuscular flaps in the pyloric antrum and the transversus abdominis muscle.¹,²,¹⁰ The canine total laparoscopic gastropexy has previously been described using two simple continuous barbed suture lines between the incised seromuscular layer of the stomach and the body wall.⁴,⁵,¹³ As in the present study, total laparoscopic barbed gastropexy has previously been performed using a single simple continuous barbed suture line in dogs, and it was considered safe and provided an intact long-term gastropexy and significantly reduced the gastropexy suturing time.⁹,¹⁴
Percutaneous stay sutures used in previous reports, to bring the pyloric antrum to the abdominal wall, were not used in any of the cases in the present study.⁵,⁹,¹³,¹⁴ Laparoscopic Babcock or Dorsey grasping forceps were used instead of the percutaneous sutures. All surgeons switched to using Babcock forceps instead of Dorsey forceps as it was easier to grasp the stomach and to hold it without any slipping. Furthermore, the Dorsey forceps need to be moved during suturing, as it impedes its realization.
Gastropexy surgery time was defined in our study between the first incision and final suture placement minus the surgery time of the other intra- and/or extra-abdominal procedure(s). Thus, a distinction was made between gastropexy time and total surgery time. The total and gastropexy surgery time in this study was shorter than that in previous studies because a few steps, such as percutaneous stay sutures placement, incision or abrasion of the seromuscular layer of the stomach and the transversus abdominis muscle, and concurrent upper gastrointestinal endoscopy, were removed; the use of one simple continuous suture also shortened the surgery time.⁴,⁵,¹³,¹⁴
Short-term postoperative complications (wound-related complication, lethargy, abdominal discomfort, and regurgitation/ vomiting) were all self-limiting and comparable with those reported in open and laparoscopic gastropexies.²⁻⁴,¹⁰,¹⁴ Two cases presented long-term complications including a granuloma and a fistula, 3 and 12 mo postoperatively, respectively. The granuloma was an incidental finding at the 3 mo postoperative ultrasound control and was not associated with any clinical sign. The dog with the fistula presented cranial abdominal discomfort and inappetence. The cause of the fistula could be barbed suture placed intraluminally during gastropexy, suture migration by normal peristaltic contractions causing extrusion of the suture into the gastric lumen or severe tissue reaction.²⁵,²⁶ At the time of the abdominal ultrasound, the fistula was observed between the subcutaneous layer of the skin and the stomach along with the presence of the suture; no extrusion of suture material into the lumen of the stomach was observed. In order to confirm that the barbed suture bites were not being placed intraluminally during gastropexy, when the stomach was grasped with the forceps, the authors felt the mucosa slip down to only suture the seromuscular layer of the stomach. Nevertheless, no concurrent upper gastrointestinal endoscopy has been performed in this study to evaluate the intraluminal region of the gastropexy site. As the barbed suture engages the tissue, it is less probable that the suture migrates. Therefore, the main hypothesis to explain the fistula is a severe tissue reaction, but its exact cause was not elucidated as no histology was performed. V-Loc 180 absorbable and V-Loc PBT nonabsorbable suturing devices were used in the present study. Nonabsorbable barbed suture (V-Loc PBT) was used in the case complicated by a long-term fistula. As all dogs with absorbable suture demonstrated permanent gastropexy, it would be advisable to use absorbable suture to decrease the risk of infection. Furthermore, the use of V-Loc 180 absorbable for intracorporeal reconstruction of the digestive tract in 242 human patients was shown to be safe and effective.²⁷
Little information is available on the impact of prophylactic gastropexy on gastric emptying and intestinal transit in dogs. Balsa et al. assessed gastrointestinal transit with wireless motility capsules in healthy dogs before and after prophylactic laparoscopicassisted gastropexy and showed that gastropexy did not alter the gastrointestinal transit in terms of gastric emptying time, small and large bowel transit time, and total transit time before and after surgery.²⁸
Focal postoperative ultrasonography showed intact gastropexies in all dogs in the present study. The minimum required follow-up time for ultrasound in the present study was 6 mo as the absorbable profile of V-Loc 180 was 180 days. There are several advantages to ultrasonographic evaluation: Unlike in experimental studies, it can be performed on live animals, there is usually no need for sedation or anesthesia, and the technique is noninvasive. Ultrasound measurements of gastric wall thickness, peristaltic contraction of the stomach, simultaneous motion of the stomach and abdominal wall during respiration, and appearance of the gastric wall layers have been used to determine efficacy of gastropexies and appear consistent and reproducible.¹,⁴,⁵,¹⁰,²⁸ Nevertheless, ultrasound has not been established to grade the quality or strength of the gastropexy.
Limitations of this study include the small sample size and its retrospective nature. Postoperative follow-up was not controlled as it would have been in a prospective study. Eight cases in this study were included prospectively and had two ultrasound examinations performed at 3 and 6 mo facilitating accurate assessment of postoperative complications. Limitations in addition to those already discussed include lack of controlled biomechanical testing. Tensile strength could not be measured in this study, but it would be interesting to test it in gastropexy performed without incision of the stomach and abdominal wall and to perform a comparison with other techniques. In vivo evaluation of permanent adhesion was performed by ultrasound examination.
Conclusion
This study suggests that prophylactic laparoscopic gastropexy may be performed with knotless unidirectional barbed suture without creating an incision on the abdominal wall or the stomach. This method is less challenging than other previously described techniques and reduces the gastropexy time compared with previous reports. Controlled biomechanical testing is indicated to further assess the efficacy and potential benefits of this procedure.
FOOTNOTES
a Applied Medical, Paris, France
b Endoflator; Karl Storz Veterinary Endoscopy, Goleta, California
c Medtronics, Minneapolis, Minnesota
d Endo Stitch; Medtronics, Minneapolis, Minnesota
e Covidien, Dublin, Ireland
f Biosyn; Medtronics, Minneapolis, Minnesota g XLSTAT-biomed statistical software; Addinsoft, New York, New York
REFERENCES
1. Rawlings CA, Foutz TL, Mahaffey MB, et al. A rapid and strong laparoscopic-assisted gastropexy in dogs. Am J Vet Res 2001;62(6): 871–5.
2. Benitez ME, Schmiedt CW, Radlinsky MA, et al. Efficacy of incisional gastropexy for prevention of GDV in dogs. J Am Anim Hosp Assoc 2013; 49(3):185–9.
3. Rivier P, Furneaux R, Viguier E. Combined laparoscopic ovariectomy and laparoscopic-assisted gastropexy in dogs susceptible to gastric dilatationvolvulus. Can Vet J 2011;52(1):62–6.
4. Spah CE, Elkins AD, Wehrenberg A, et al. Evaluation of two novel selfanchoring barbed sutures in a prophylactic laparoscopic gastropexy compared with intracorporeal tied knots. Vet Surg 2013;42(8):932–42.
5. Coleman KA, Adams S, Smeak DD, et al. Laparoscopic gastropexy using knotless unidirectional suture and an articulated endoscopic suturing device: seven cases. Vet Surg 2016;45(S1):O95–O101.
6. Runge JJ, Mayhew PD, Rawlings CA. Laparoscopic-assisted and laparoscopic prophylactic gastropexy: indications and techniques. Compend Contin Educ Vet 2009;31(2):58–65.
7. Runge JJ, Mayhew PD. Evaluation of single port access gastropexy and ovariectomy using articulated instruments and angled telescopes in dogs. Vet Surg 2013;42(7):807–13.
8. Hardie RJ, Flanders JA, Schmidt P, et al. Biomechanical and histological evaluation of a laparoscopic stapled gastropexy technique in dogs. Vet Surg 1996;25(2):127–33.
9. Mathon DH, Dossin O, Palierne S, et al. A laparoscopic-sutured gastropexy technique in dogs: mechanical and functional evaluation. Vet Surg 2009;38(8):967–74.
10. Mayhew PD, Brown DC. Prospective evaluation of two intracorporeally sutured prophylactic laparoscopic gastropexy techniques compared with laparoscopic-assisted gastropexy in dogs. Vet Surg 2009;38(6): 738–46.
11. Arbaugh M, Case JB, Monnet E. Biomechanical comparison of glycomer 631 and glycomer 631 knotless for use in canine incisional gastropexy. Vet Surg 2013;42(2):205–9.
12. Imhoff DJ, Cohen A, Monnet E. Biomechanical analysis of laparoscopic incisional gastropexy with intracorporeal suturing using knotless polyglyconate. Vet Surg 2014;44(Suppl 1):39–43.
13. Coleman KA, Monnet E. Comparison of laparoscopic gastropexy performed via intracorporeal suturing with knotless unidirectional barbed suture using a needle driver versus a roticulated endoscopic suturing device: 30 cases. Vet Surg 2017;46(7):1002–7.
14. Takacs JD, Singh A, Case JB, et al Total laparoscopic gastropexy using 1 simple continuous barbed suture line in 63 dogs. Vet Surg 2017;46(2): 233–41.
15. Mayhew PD. Laparoscopic and laparoscopic-assisted cryptorchidectomy in dogs and cats. Compend Contin Educ Vet 2009;31(6):274–81.
16. Mayhew PD. Techniques for laparoscopic and laparoscopic-assisted biopsy of abdominal organs. Compend Contin Educ Vet 2009;31(4): 170–6.
17. Runge JJ, Mayhew PD, Case JB, et al. Single-port laparoscopic cryptorchidectomy in dogs and cats: 25 cases (2009-2014). J Am Vet Med Assoc 2014;245(11):1258–65.
18. Verdaasdonk EG, Dankelman J, Lange JF, et al Transfer validity of laparoscopic knot-tying training on a VR simulator to a realistic environment: A randomized controlled trial. Surg Endosc 2008;22(7):1636–42.
19. Ehrhart NP, Kaminskaya K, Miller JA, et al. In vivo assessment of absorbable knotless barbed suture for single layer gastrotomy and enterotomy closure. Vet Surg 2013;42(2):210–6.
20. Covidien. V-LOC 90 and V-LOC 180 absorbable wound closure devices step-by-step guide to dermal wound closure applications. http://www.medtronic.com/content/dam/covidien/library/us/en/product/ wound-closure/v-loc-wound-closure-devices-guide-to-wound-closureapplications.pdf.
21. Wacker C, Weber T, Tanno F, et al Ultrasonographic evaluation of adhesion induced by incisional gastropexy in 16 dogs. J Small Anim Pract 1998;39(8):379–84.
22. Gutt CN, Oniu T, Schemmer P, et al. Fewer adhesions induced by laparoscopic surgery? Surg Endosc 2004;18(6):898–906.
23. Einarsson JI, Grazul-Bilska AT, Vonnahme KA. Barbed vs standard suture: randomized single-blinded comparison of adhesion formation and ease of use in an animal model. J Minim Invasive Gynecol 2011;18(6):716–9.
24. Thubert T, Pourcher G, Deffieux X. Small bowel volvulus following peritoneal closure using absorbable knotless device during laparoscopic sacral colpopexy. Int Urogynecol J 2011;22(6):761–3.
25. Milovancev M, Weisman DL, Palmisano MP. Foreign body attachment to polypropylene suture material extruded into the small intestinal lumen after enteric closure in three dogs. J Am Vet Med Assoc 2004;225(11):1713–5.
26. Postlethwait RW. Five year study of tissue reaction to synthetic sutures. Ann Surg 1979;190(1):54–7.
27. Lee SW, Kawai M, Tashiro K. et al. Laparoscopic gastrointestinal anastomoses using knotless barbed absorbable sutures are safe and reproducible: a single-center experience with 242 patients. Jpn J Clin Oncol 2016;46(4):329–35.
28. Balsa IM, Culp WTN, Drobatz KJ, et al. Effect of laparoscopic-assisted gastropexy on gastrointestinal transit time in dogs. J Vet Intern Med 2017; 31(6):1680–5.
 .
.