If you need to print this PDF or save for later
click below to download your copy.
[button size=”medium” style=”secondary” text=”Download PDF” link=”https://vetovationnew.instawp.xyz/wp-content/uploads/2019/12/PlasmappSterlink-EN-14-page-brochure.pdf” target=”none”]
ON-DEMAND
Low-Temperature Plasma Sterilization
Introducing STERLINK
A low-temperature gas plasma sterilizer to sterilize a wide range of medical devices
The STERLINK®FPS–1Ss Plus gas sterilization veterinary system is a low temperature plasma sterilizer to inactivate microorganisms for a broad range of metal and nonmetal medical devices and surgical instruments at low temperature. This product is reliable and provides a variety of methods of sterilization.
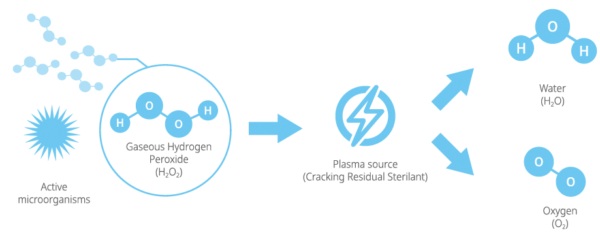
STERLINK®can sterilize medical devices by diffusing hydrogen peroxide vapor into the chamber or pouch. It rapidly sterilizes medical instruments and materials without leaving toxic residues. All stages of the plasma sterilization cycle does not damage compatible instruments which are sensitive to heat and moisture.
This sterilizer can be used for metal and non-metal medical devices and can sterilize instruments with high lumen characteristics and micro sized equipments.
It consistently provides the Sterility Assurance Level (SAL) of 10-6, as defined by U.S. Food and Drug Administration (FDA) and international standards, only when used within the materials and geometric requirements given.
The devices have been pre-validated to the SAL of 10·5 based upon high resistance conditions, including lumens within the claim lengths and mated surfaces.
STERLINK® Overview

Fast Sterilization
- Smart Ready™ allows auto detection of moisture and pre-drying
- Just touch and start sterilization
- Automatic mode selection with individual barcode on pouch/cartridge

Convenient POWER SAVING
- Sleep mode (Heater off)
- Screen saver for LCD display

Eco-Friendly
- Decomposing residual H202 into WATER and OXYGEN
- Equipped with 03 purifying filter

Perfect DISINFECTION
- Improved sterile reliability with HEPA filter
- Easily replaceable
Different Modes
STERPACK® (Pouch Mode)
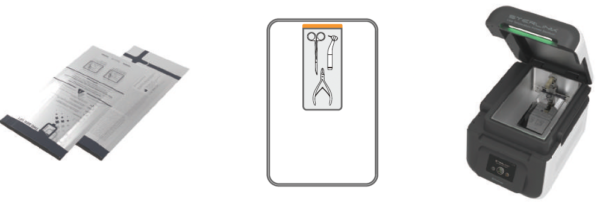
World first pouch-type sterilization during 4 minutes is possible with the patented direct sterilant injection technology from Plasmapp.
- Vacuum sealed pouch can be stored up to 6 months in sealed sterile condition.
- STERPACK®sterilization does not require chamber cleaning as it leaves no residue in the chamber.
- Vacuum condition visualizes the sterile condition and prevents second contamination.
- Sterilant is stored safety inside the pouch which prevents any hazard from chemical exposure.
POUCH MODE
- Sterilization Method: Pouch mode in FPS-15s Plus
- Dimension: 135mm X 280mm
- Sterilant: H202 58%-59.5% (01 cc/cell)
- SR™ / SC” Cycle: 3-6 min
- Sterilization Cycle: 4 min
- Overall: 7-10 min
STERPACK® Plus (Pouch Plus Mode)
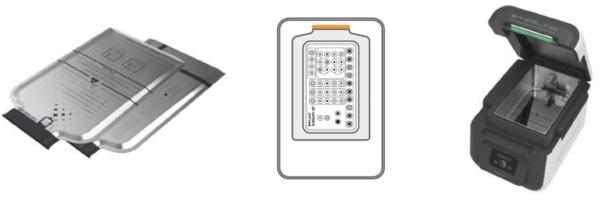
Large capacity pouch reflecting market needs.
- Larger Volume
- Load Diversity
- Implant kit
- Clinical surgery kit
- Quick Cycle
- 8 min sterilization process time
- Perfect vacuum seal
- 6 months preservation period
POUCH Plus MODE
- Sterilization Method: Pouch mode in FPS-15s Plus
- Dimension: 240mm X 410mm
- Sterilant: H202 58%-59.5% (0.3 cc/cell)
- SR™/ SC™ Cycle: 6-10 min
- Sterilization Cycle: 8 min
- Overall: 14-18 min
STERLOAD® (Chamber Mode)
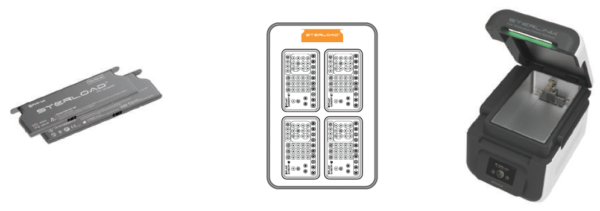
It is sterilized immediately in 15 minutes using a large chamber of 14 liters to increase user efficiency.
- Safe structure design contains sterilization agent inside cartridge to prevent exposure risk.
- Ergonomic design facilitates chamber mounting.
- No residue after sterilization to maintain clean chamber.
- Using 1 cartridge per cycle for economic usage.
- Using Tyvek“ pouch protects sterilized medical tools from dust.
CHAMBER MODE
- Sterilization Method: Chamber mode in FPS-15s Plus
- Dimension: 264mm X 410mm (H 125 mm)
- Sterilant: H202 58%-59.5% (0 9 cc/cell)
- SR'”/ SC” Cycle: 21 min
- Sterilization Cycle: 15 min
- Overall: 36 min
Plasma Sterilization Cycle
Flexible Sterilization modes depend on the capacity
The STERLINK®FPS-15s Plus sterilizer provides a smart sterilization process that combines Smart Ready (SR™) and sterilization process and Smart Complete (SC™) to ensure sterilization efficiency, reliability and user safety. The gas plasma sterilizer system is designed to operate only with the sterilant cassettes of STERPACK®, STERPACK® Plus and STERLOAD®. Each cassette has individual barcode which is scanned by the sterilizer to start the sterilization cycle automatically according to the barcode identifying the type of the cassettes.
The SR™ process is provided to measure residual moisture left on the medical devices and to perform optimized heating and drying process according to the analysis.
The sterilization process consists of the two consecutive and equal phases, and the critical process parameters in each phase are identical. The validation of the sterilization process is performed by using the half-cycle overkill method to demonstrate the 10-6 SAL. The following table provides a brief description for each cycle.
[table id=14 /]
Sterilization Performance
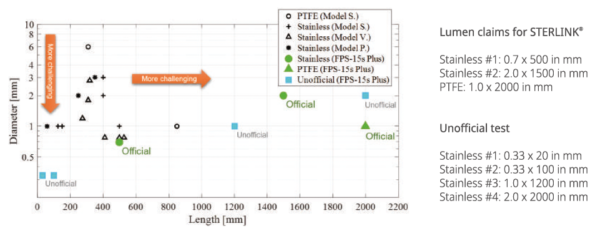
The lumen test is mandatory for invasive sterilization. The lumen structure consists of long tube connected with BI container. It is more difficult to be sterilized against the lumen with longer length and smaller diameter. The STERLINK®FPS-15s Plus has been tested with more challenging lumen structure.
Sterilization Verification
We guarantee the best sterilization standard in reference to SAL of 106. After the test with BI inside the lumen, you can check whether the sterilization is reliable by cultivating the BI in the incubator. All the sterilization test report are certified by Korean MFDS and CE/MOD.

STERLINK® Sterilization Process


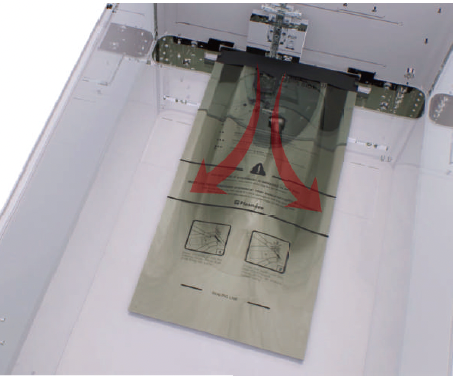
Direct Injection Technology
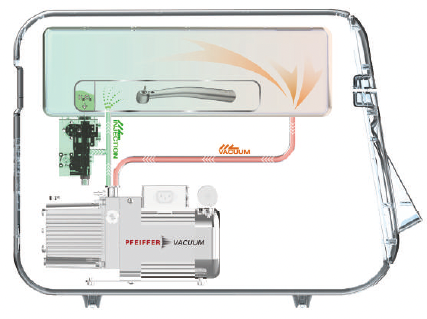
Patented Vaporizer Technology
Hydrogen Peroxide Gas Sterilization Requires 4 Key Elements
- Sterilant – Secure retention reliability and user safety
- Temperature Control – High speed temperature control using forced convection and thermal transfer
- Vacuum Condition – Achieving 99% or more vacuum condition
- Vaporizer – Patented Vaporizer specialized for direct injection
These 4 key elements are the core basis for achieving optimal Hydrogen Peroxide Gas Sterilization
Plasma Sterilization Reliability
Barcode Tracking
Each Barcode on STERPACK®, STERPACK®Plus and STERLOAD®allows for sterilization record tracking for individual patients. Barcode for STERPACK®& STERPACK®Plus & STERLOAD®Tracking Production Information included to assure STERPACK®& STERPACK®Plus & STERLOAD®validity. It helps prevent the use of expired cassettes. Also, barcode on cassettes find sterilization mode by itself.
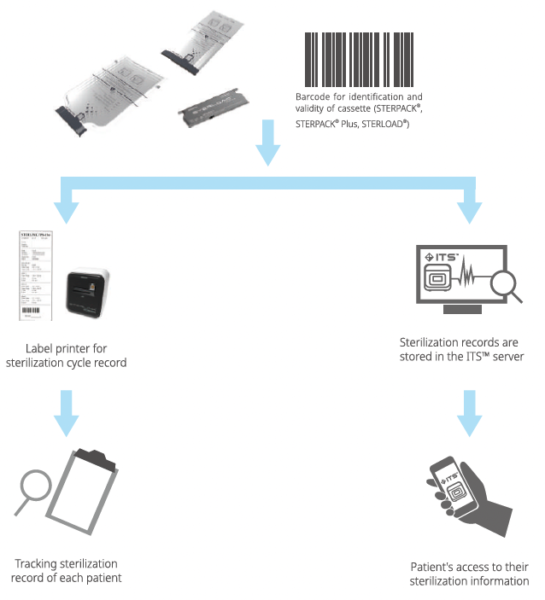
ITS™-Instrument Tracking System
Instrument Tracking System is a real-time sterilization monitoring system. Available as an analysis of actual usage data of STERPACK®, STERPACK®Plus and STERLOAD® as a remote device diagnostic tool. Provide advanced services that deliver accurate data analysis and immediate delivery to end users.
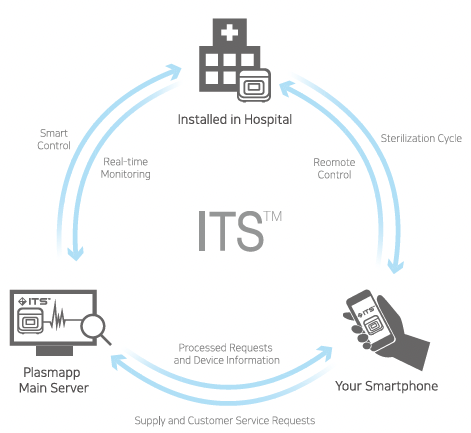
- ITS™ allows real-time monitoring which enables before service and worry-free usage because problems are tracked and resolved remotely.
- ITS™ securely records sterilization data in Plasmapp’s main server.
- ITS™ monitors operation status of every sterilizer for on-time and accurate maintenance care.
- ITS™ provides remote software update to maintain STERLINK®with most updated software.
- Easy to use ITS™ mobile application allows direct access to sterilization records and sterilizer control.
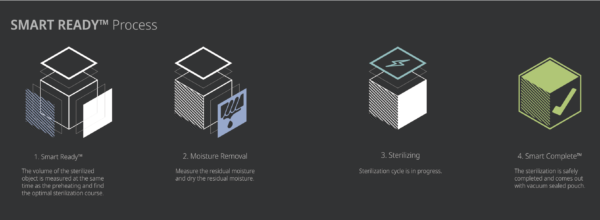
SMART READY™/ SMART COMPLETE™
Starting sterilization cycle
- Check whether the door is closed when the load has been properly placed in the chamber.
- Touch “Sterilize” icon to start sterilization cycle.
- The sterilizer automatically scans the barcode printed on the cassette to check validity of the loaded cassette and determine the operation mode.
- The sterilizer automatically check the door status and start the sterilization cycle.
Cycle in progress and completed
The drying process and sterilization process will be initiated after measuring the load condition at the SR™ process.
When the gas sterilization veterinary process is successfully completed, the smart complete (SC™) process is performed in order to ensure that there is no residual sterilant left on the sterilized instruments. After the relatively short SC™ process, the sterilization cycle is finished with summary of the cycle. Press the confirm switch to return to ready state.
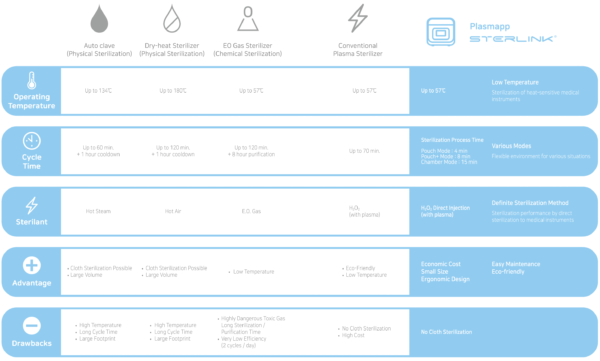
Comparison of sterilizers with STERLINK®
Specification
[table id=15 /]
Dimension (unit: mm)

Sterilant cassettes, Consumables and Accessories
Sterilant cassettes

STERPACK®
Impermeable pouch containing sterilant for POUCH mode
- Size: 135mm x 280mm
- Material: PP/NY
- Sterilant: Hydrogen peroxide (concentration: 58%-59.5%)
- 1 cycle per pouch

STERPACK®Plus
Impermeable pouch containing sterilant for POUCH Plus mode
- Size: 240mm x 410mm
- Material: PP/NY
- Sterilant: Hydrogen peroxide (concentration: 58%-59.5%)
- 1 cycle per pouch

STERLOAD®
Cassette containing sterilant for CHAMBER mode
- Size: 135mm x 42mm x 7mm
- Material: PC, PP
- Sterilant: Hydrogen peroxide (concentration: 58%-59.5%)
- 1 cycle per cassette
Accessories

Special Cart
Cart with locking wheel
- Size: 483mm x 660mm x 603mm
- Weight: 37kg

Label-Printer
External thermal printer
- Size: 120mm x 102mm x 146mm
- Weight: 0.5kg
- Thermal paper width: 56mm

STERSEAL®
Rotary sealer by HAWO
- Size: 505mm x 255mm x 145mm
- Weight: 12kg
- CE, ISO 11607
- Preset STERPACK® sealing mode

Label Sticker Roll
Label sticker roll for label-printer
- Roll width: 56mm
- Roll length: 49 labels

Chemical Indicator Tape
Chemical indicator for sterilization cycle monitor
- Tape width: 19mm
- Length: 55m
- Expiration date: 12 months after manufacturing date

Chemical Indicator Strip
Chemical indicator for sterilization cycle monitor:
- Size: 16mm x 100mm
- Expiration date: 12 month are manufacturing date
- 250 strips per box
Consumables

Tyvek® pouch (W 100)
Device sterile packaging pouch for CHAMBER mode
- Pouch width: 100mm
- Total length: 400mm
- Material: Tyvek® and Easy-Peel film
- 1 cycle per pouch

Tyvek® pouch (W 200)
Device sterile packaging pouch for CHAMBER mode
- Pouch width: 200mm
- Total length: 400mm
- Material: Tyvek® and Easy-Peel film
- 1 cycle per pouch

Tyvek® pouch (W 300)
Device sterile packaging pouch for CHAMBER mode
- Pouch width: 300mm
- Total length: 400mm
- Material: Tyvek® and Easy-Peel film
- 1 cycle per pouch
Plasmapp Co., Ltd.
Plasmapp is an innovative manufacturing company specializing in plasma technology. Based on the core technology of KAIST plasma physics laboratory, we are creating a market with differentiated products in the medical and industrial plasma device industries. Developed for medical markets, the world’s first direct injection technology is certified and commercialized.
In the industrial plasma device market, we have developed and commercialized a plasma source (LJPS®) capable of stable surface treatment at atmospheric pressure, thus improving the productivity of the secondary battery manufacturing process.
Think Plasma, Think Plasmapp!
Plasmapp Co.,Ltd. Values innovation, diligence, creativeness and technical know-how to create smart plasma applications to satisfy every needs of plasma-tech industry. It is our mission to provide high quality and cutting-edge plasma solutions.
Best regards,
CEO of Plasmapp
Youbong Lim